
Recombinant Human Granulocyte-Macrophage Colony Stimulating Factor
产品名称: Recombinant Human Granulocyte-Macrophage Colony Stimulating Factor
英文名称: Granulocyte/Macrophage Colony-Stimulating Factor, CSF-2, MGI-1GM, Pluripoietin-α
产品编号: IC-102-03
产品价格: null
产品产地: 美国
品牌商标: XY-Bioscience
更新时间: 2023-08-17T09:55:27
使用范围: > 98 % by SDS-PAGE and HPLC analyses.
- 联系人 : 徐经理
- 地址 : 上海市闵行莘庄工业区春东路508号A1-2F
- 邮编 : 200612
- 所在区域 : 上海
- 电话 : 152****8802 点击查看
- 传真 : 点击查看
- 邮箱 : shxysw02@163.com
- 二维码 : 点击查看
Recombinant Human Granulocyte-Macrophage Colony Stimulating Factor
| Synonyms | Granulocyte/Macrophage Colony-Stimulating Factor, CSF-2, MGI-1GM, Pluripoietin-α |
| Accession | P04141 |
| GeneID | 1437 |
| Source | Escherichia coli. |
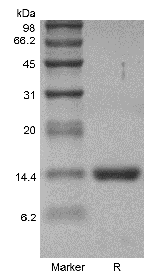
| Molecular Weight | Approximately 14.5 kDa, a single non-glycosylated polypeptide chain containing 127 amino acids. |
| Quantity | 5µg/100µg/1mg |
| AA Sequence | APARSPSPST QPWEHVNAIQ EARRLLNLSR DTAAEMNETV EVISEMFDLQ EPTCLQTRLE LYKQGLRGSL TKLKGPLTMM ASHYKQHCPP TPETSCATQI ITFESFKENL KDFLLVIPFD CWEPVQE |
| Purity | > 98 % by SDS-PAGE and HPLC analyses. |
| Biological Activity | Fully biologically active when compared to standard. The ED50 as determined by a cell proliferation assay using human TF-1 cells is less than 0.1 ng/ml, corresponding to a specific activity of > 1.0 × 107 IU/mg. |
| Physical Appearance | Sterile Filtered White lyophilized (freeze-dried) powder. |
| Formulation | Lyophilized from a 0.2 μm filtered concentrated solution in PBS, pH 7.4. |
| Endotoxin | Less than 0.01 EU/μg of rHuGM-CSF GMP as determined by LAL method. |
| Reconstitution | We recommend that this vial be briefly centrifuged prior to opening to bring the contents to the bottom. Reconstitute in sterile distilled water or aqueous buffer containing 0.1 % BSA to a concentration of 0.1-1.0 mg/mL. Stock solutions should be apportioned into working aliquots and stored at ≤ -20 °C. Further dilutions should be made in appropriate buffered solutions. |
| Stability & Storage | Use a manual defrost freezer and avoid repeated freeze-thaw cycles. - 12 months from date of receipt, -20 to -70 °C as supplied. - 1 month, 2 to 8 °C under sterile conditions after reconstitution. - 3 months, -20 to -70 °C under sterile conditions after reconstitution. |
| Usage | This GMP product can be for research use or further manufacturing use. |
| Quality statement | The manufacture and testing of this product is in compliance with ICH Q7a guidelines. |
| Reference | 1. Wang JM, Chen ZG, Colotta F, et al. 1988. Behring Inst Mitt: 270-3. 2. 1989. N Engl J Med, 320: 253-4. 3. Nissen-Druey C. 1989. Nouv Rev Fr Hematol, 31: 99-101. 4. Eager RandNemunaitis J. 2005. Mol Ther, 12: 18-27. 5. Tran T, Fernandes DJ, Schuliga M, et al. 2005. Br J Pharmacol, 145: 123-31. |
| Background |
Granulocyte-Macrophage Colony Stimulating Factor (GM-CSF) is secreted by a number of different cell types (including activated T cells, B cells, macrophages, mast cells, endothelial cells and fibroblasts) in response to cytokine or immune and inflammatory stimulation. It was initially characterized as a growth factor that can support the in vitro colony formation of granulocyte-macrophage progenitors and has functions of stimulates the growth and differentiation of hematopoietic precursor cells from various lineages. GM-CSF has also been reported to have a functional role on non-hematopoietic cells and can induce human endothelial cells to migrate and proliferate. Additionally, it can stimulate the proliferation of a number of tumor cell lines, including osteogenic sarcoma, carcinoma and adenocarcinoma cell lines. Human GM-CSF shares 54 % sequences identity with mouse GM-CSF, but has no biological effects across species. GM-CSF is used as a medication to stimulate the production of white blood cells following chemotherapy and has also recently been evaluated in clinical trials for its potential as a vaccine adjuvant in HIV-infected patients. |
